Any waste gas purification unit is a chemical process unit
Purifying off-gas, or waste air, essentially means separating the stream into a large stream of clean air and another smaller stream that contains all the undesired substances in a concentrated form. In most cases, these undesired molecules are not just being deposited, but have to be transformed to harmless substances, or recycled.
Examples
- Physisorption, as it occurs on activated carbon or on zeolites, is the formation of complex bonds.
- Absorption in a scrubber is a dissolution in a solvent system.
- Several types of oxidations take place in combustion units, oxidative scrubbers or on adsorbents which are solid-state oxidants.
While the composition of the waste gas influences how the waste gas purification unit will work, its operating conditions will shift the chemical equilibrium and thereby the resulting emissions.
Off the beaten track – how to design a waste gas purification unit under unusual conditions
The standard procedure for selecting a waste gas purification method relies on the gas volume to be treated, its conditions and VOC concentrations. Sometimes, though, the conditions are outside of known empirical values. Some examples might be:
- For adsorption units: Moisture contents higher than the typical 50 – 60% r. h. can affect adsorption on activated carbon or zeolite. High relative humidity levels do not only occur from „wet“ sources, but also if room air is led through outdoor channels in a four-season climate.
- Changing temperatures, concentrations or waste gas volumes in any type of waste gas purification can lead to sudden breakthrough – whether the unit is a bio filter, a scrubber or an adsorption. This is typical for waste gas from batch production or single-shift operations.
- Contaminants can influence each other. The presence of one solvent can, for example, raise the vapor pressure of another solvent in an aqueous scrubber or reduce its adsorption capacity on a solid sorbent far beyond what is expected within an ideal model. This is especially tricky with multi-purpose plants – you will never know exactly what waste gas composition to expect.
Some systems show unusual behaviour, e. g. extreme changes at rising temperature, or adsorption-desoprption hysteresis which can effectively disable a filter bed. Many silica gels show adsorption hysteresis, with the shape of the hysteresis loop changing over time. Some adsorptives, like acetone, switch their isotherm typewith rising temperature. - Sometimes the waste gas is just nasty: It contains polymerizable or otherwise unstable, reactive or corrosive gases. One example would be styrene or methyl methacrylate in waste gas from coating or adhesive production.
Simulation is possible as long as the waste gas purification is a steady state process and thermodynamic model data are available. For real-world systems consisting of three or more components this is rarely the case.
Fast workarounds
The legitimate approach in this situations would be to either try out the suggested waste gas purification technique on a pilot scale, or to measure the missing thermodynamic data, build a thermodynamic model and then simulate the process, which would still have to be tested before implementation. Often, though, it is possible to check for „killer criteria“ or do a rough feasibility analysis with chemical methods. This usually does not take more than a few hours and can save weeks of work. The case studies on the following pages show a few examples of this type of analysis.
Case studies
Case Study I: Styrene Emissions from a Craft Business
A family-owned business produces bespoke tanks and basins from fiberglass and polyester resin by hand-rolling. According to law, styrene emissions from the ventilating system have to be reduced to 20 mg/m³, but actually the odor threshold levels for styrene are much lower – around 0,3 mg/m³ – and complaints from the residents in the mixed use area occur frequently. For this small business the cost of standard waste gas purification procedures could be critical for survival.
Case Study II: Design of an Adsorption-based Smoothing Filter (Ripple Filter)
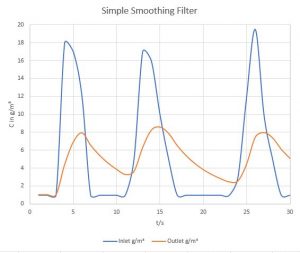
To avoid having to construct a waste gas purification unit to fit the largest concentrations of irregular VOC loads, it has often been attempted to install a passive ripple smoothing filter, i. e. an adsorbent bed that would adsorb high concentrations of volatiles and release them during times of lower loads. But how realistic is such a smoothing filter, especially its passive, uncontroled variety?
Case Study III: Retrofit of an Existing Incineration Plant With a Concentrator
A regenerative thermal oxidation unit working below its concentration capacity, additional waste gas streams – no problem, just add a concentrator to the system. But how do you handle it if the additional waste gas comes from a multi-purpose plant with extremely varying VOC compositions?
Case Study IV: Oxidative Scrubbers – How to, and When to scrub VOC From Air
Add some oxidising agent (like sodium hypochlorite or hydrogen peroxide) into your scrubber and you’re done – you end up with CO2 and salt water. But will this work? How to find out whether it will work, and will be a good alternative.
Case Study V: Natural Zeolites as Filter Material for Ammonia – Is It a Way to Go?

Natural zeolites are often used to adsorb ammonia – as an additive for bedding in stables and as a feed additive, especially for ruminants. Besides, the price for natural zeolite can be temptingly low. But does it do its job as an odor control filter in animal husbandry?
Case Study VI: Autoignition in Adsorbing Filters
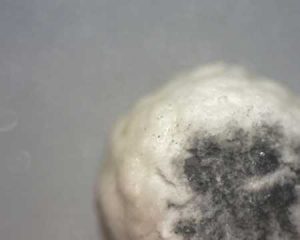
A thermal runaway in an adsorption filter can run to very high temperatures, even if the adsorbent is the per se incombustible zeolite. After such an incident, the company not only needed a retrofit and operational concept to prevent further such catastrophic incidents, but also had to convince their insurance company that their retrofitted waste gas purification unit was now safe.