In his stable, a livestock owner ran a ventilation which in winter blew out 30.000 m³/h STP of ammonia-laden air. In summer, the amount of air could go up to 150.000 m³/h STP, depending on the weather. Ammonia concentrations were between 15 and 50 mg/m³, the human detection threshold for ammonia odor being well below 1 mg/m³ [1]. Besides, the waste air could be rather warm – up to 3 °C above ambient temperature in summer.
Ammonia abatement technologies
Ammonia can be removed from air streams in a number of ways. The choice depends on the concentration of gaseous ammonia, and whether this has to be reduced or, as was the case here, only the irritating ammonia odor has to be removed.
- Scrubbing and catalytic technologies are expensive choices. They would be used when a true reduction of ammonia concentration has to be achieved.
- Adsorption by pretreated activated carbon would be another possibility. In the case of this stable, though, the high and changeable temperatures as well as the high, fluctuating air flow would lead to poor utilization of the activated carbon. Though the upfront cost of this technique is intriguingly low, costs for the expensive impregnated activated carbon would add up mercilessly.
- Sometimes there is the chance to mask an odor by adding a masking substance to the air stream (something with an odor of its own which covers the smell of ammonia), either with or without massive addition of water vapor. Condensed water droplets act as a solvent for ammonia. Depending on weather factors such as wind, temperature, solar radiation etc. the ammonia-bearing water vapor can condense somewhere on the ground instead of harassing neighbour’s noses. This is not a reliable method, though, especially in those cases in which neighbour’s complaints are the main part of the problem.
- Biofiltration can also be used to reduce ammonia odor. The more effective, but also more expensive variation of this technique is a bioscrubber, or a biotrickling filter. The ammonia is dissolved in water as a first step, bacterial degradation takes place in aqueous solution. Dry biofiltration on a bed of bark mulch, heather or some other solid organic material combines uptake and biodegradation of ammonia into one step. These filters are highly sensitive to cold, dryness and a number of other disruptive influences. Sometimes no degradation takes place and the odor is only masked by the odor of the mulch itself, so that after a few weeks, the biofilter will seemingly stop to function (though it never really functioned in the first place).
Natural zeolite – from waste gas filtration material to fertilizer?

The livestock owner had learned during his internet research that natural zeolites can adsorb ammonia. His idea was to install a filter with fine zeolite gravel and to use the mineral as a fertilizer once its adsorption capacity for ammonia was exhausted. Zeolites are known to improve many types of soil, due to their porosity, their water binding and ion exchange properties. He contacted me to roughly calculate how large and expensive such a filter would be, in which the material would have be exchanged four to six times a year.
Natural zeolite and ammonia – some background
The largest part of natural zeolite is actually used for ammonia removal, but in a completely different setting – in aquaculture. Ammonia from the fecal matter of fish is partially adsorbed on a submersed filter of zeolite gravel. Some ammonia will be present in its ammonium form. This is absorbed into the zeolite via an ion exchange mechanism. The zeolite acts as a temporary buffer for the ammonia/ammonium load which is metabolized by bacteria conveniently settled on the porous, mineral-rich zeolite.
Adsorption of gaseous ammonia onto zeolite – mostly clinoptilolite – is a totally different matter. No ion exchange can take place under gaseous conditions, and thus the adsorption capacity for ammonia is much smaller than in aqueous solution. There is normally no biofilm with a sufficient metabolic capacity to dispose of ammonia under dry conditions. It is an „adsorb and discharge-process“ only.
Filter design
The scientific literature contains a number of publications in which the adsorption capacity of natural zeolitites – mostly clinoptilolites – have been tested against their capacity to adsorb ammonia. This adsorption capacity is so poor that any functioning filter would be huge, with an enormous pressure drop.
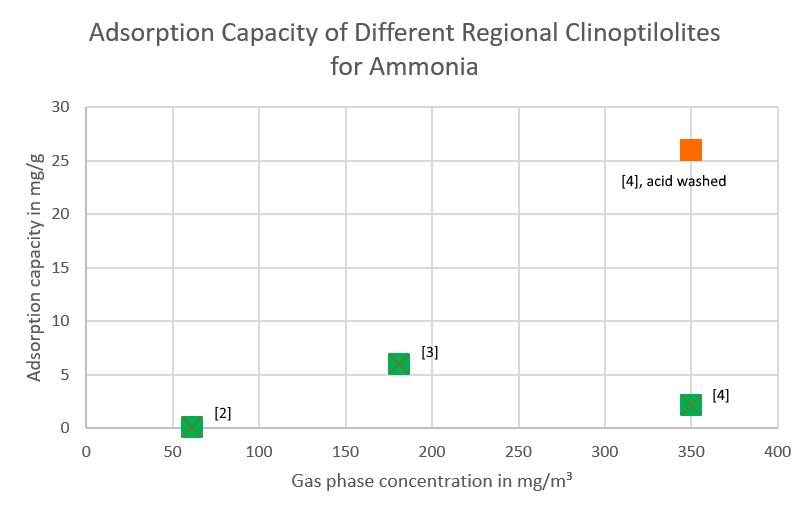
The most local zeolite would be no. 4, from Sovakia. Its adsorption capacity is reported to be 2,2 mg/g (i. e. 0,22% on a weight basis) at 350 mg of ammonia. For the lower concentrations present, an interpolated adsorption capacity of 0,4 – 1 mg/g can be expected. But even were the zeolite treated with sulfuric acid as was done in the study and the full capacity of 26 mg/g achieved, the filter would have to contain 250 tons of zeolite for 90 days. Even with modularized filters to adapt to the changing air flow, five filters, each with a pressure drop of around 12,000 Pa (twelve thousand) and an abutting area of 47 m² each.
Even though every resercher writes in his paper that „… the results show that this material promises to be an effective filter medium for ammonia“, a simple calculation shows that under real conditions, this can not be implemented. Even with benchscale test results equal to the best published results, such a filter would still be prohibitively expensive and use way too much energy. Therefore, no tests were performed in this case.
[1] http://www.ccac.ca/Documents/Standards/Ammonia.pdf
[2] „Clinoptilolite Adsorption Capability of Ammonia in Pig Farm“ Xue Li et al., Procedia Environmental Sciences 2 (2010) 1598–1612
[3] „Removal of Ammonia from Air, using Three Iranian Natural Zeolites“. H. Asilian et al., Iranian J Publ Health, Vol. 33, No. 1, pp.45-51, 2004.
[4] „Removal of ammonia from waste air streams with klinoptilolite in its natural and treated forms.“ K. Ciahotný et al., Adsorption (2006) 12:219-226.